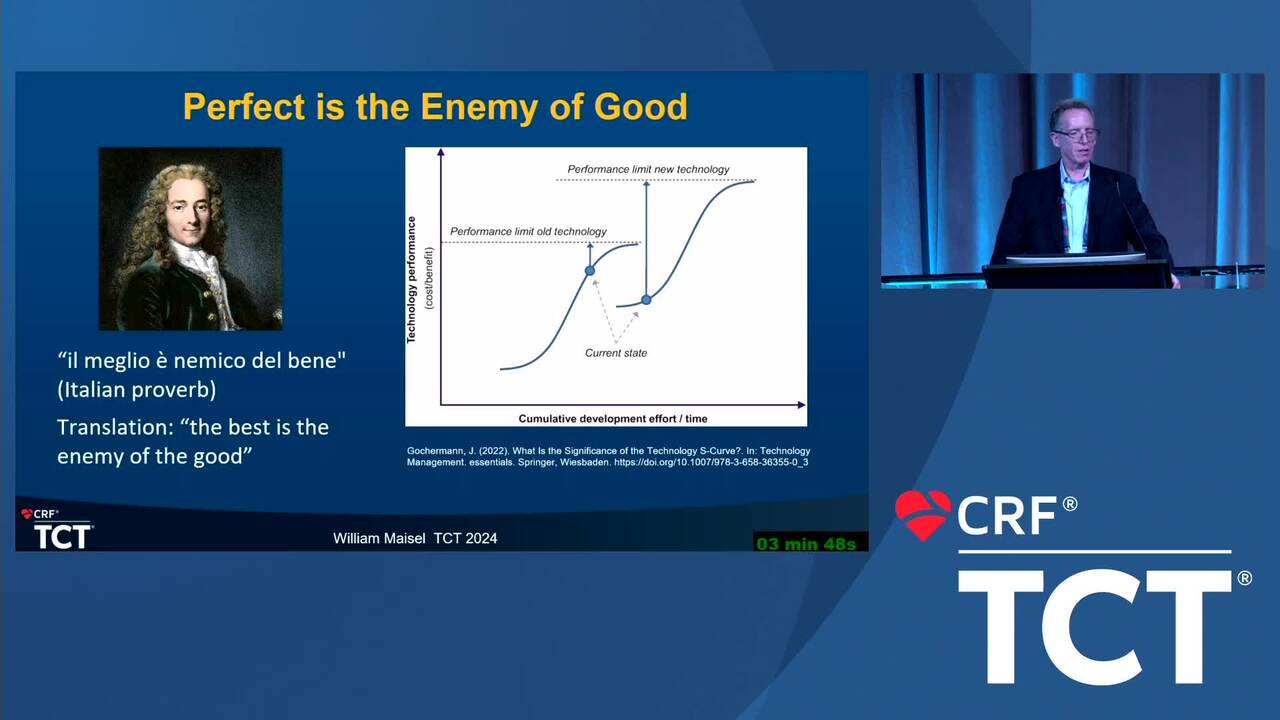
Reflections on Innovation From a Recent FDA Leader
8 min.
Breakthrough device designation: Beyond the Bragging Right
51 min.
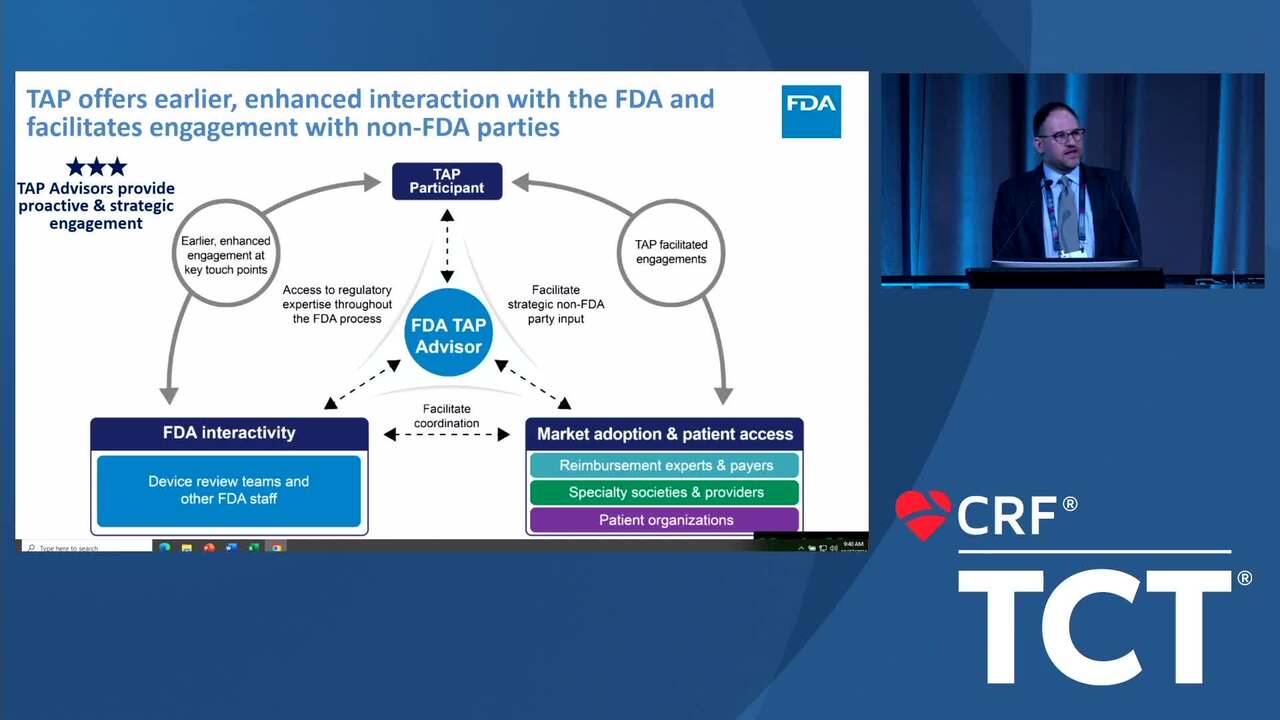
Total Product Lifecycle Advisory Program (TAP): FDA's Medical Device Accelerator
12 min.
Tricuspid Valve Therapies - Current Status and Next Steps: Post-Market Considerations - How Can We Ensure Rational Diffusion of TTVI, Optimal Training and Maintenance of Quality?
21 min.
Tricuspid Valve Therapies - Current Status and Next Steps: Lessons From the Initial FDA Trials
37 min.
Do We Need Placebo-Controlled Regulatory Trials: No, We Have Enough Evidence Already
16 min.
Patient-Reported Outcomes - Incorporation of PROs in Composite Endpoints: Is the Win Ratio the Optimal Approach?
29 min.
Patient-Reported Outcomes- Interpretation of PROs: Understanding Validity and Clinically-Important Differences
9 min.
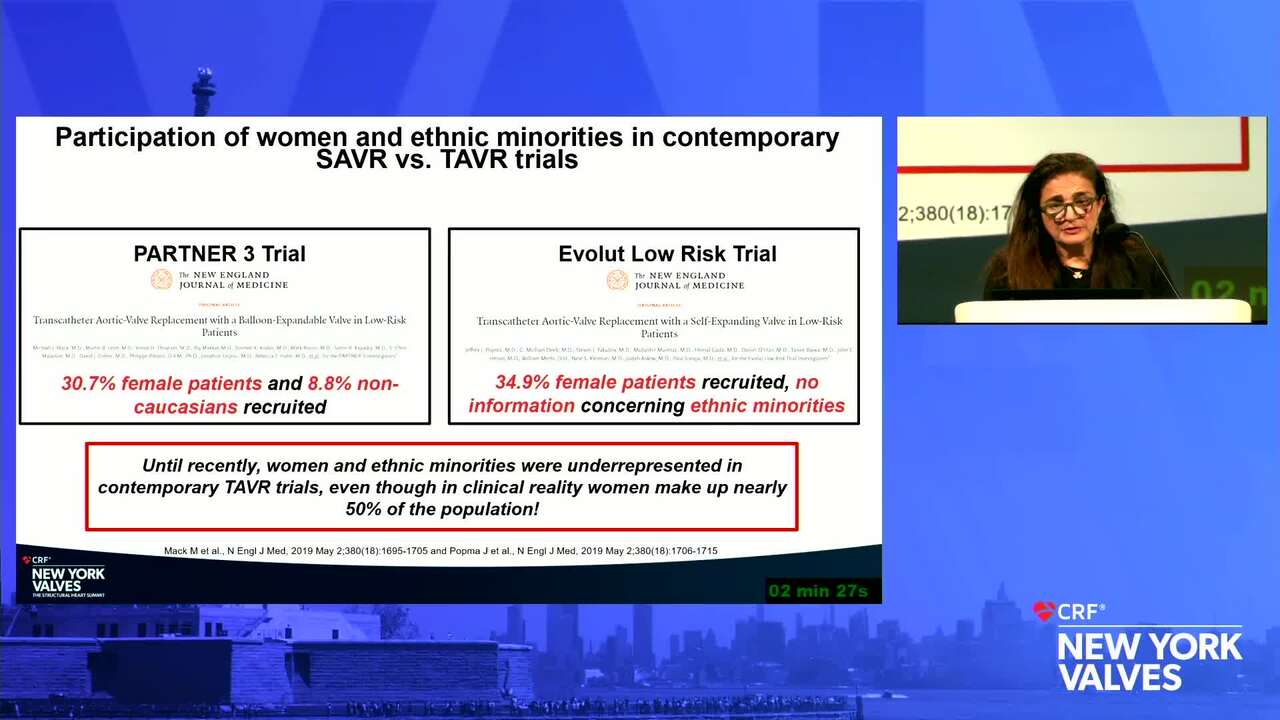
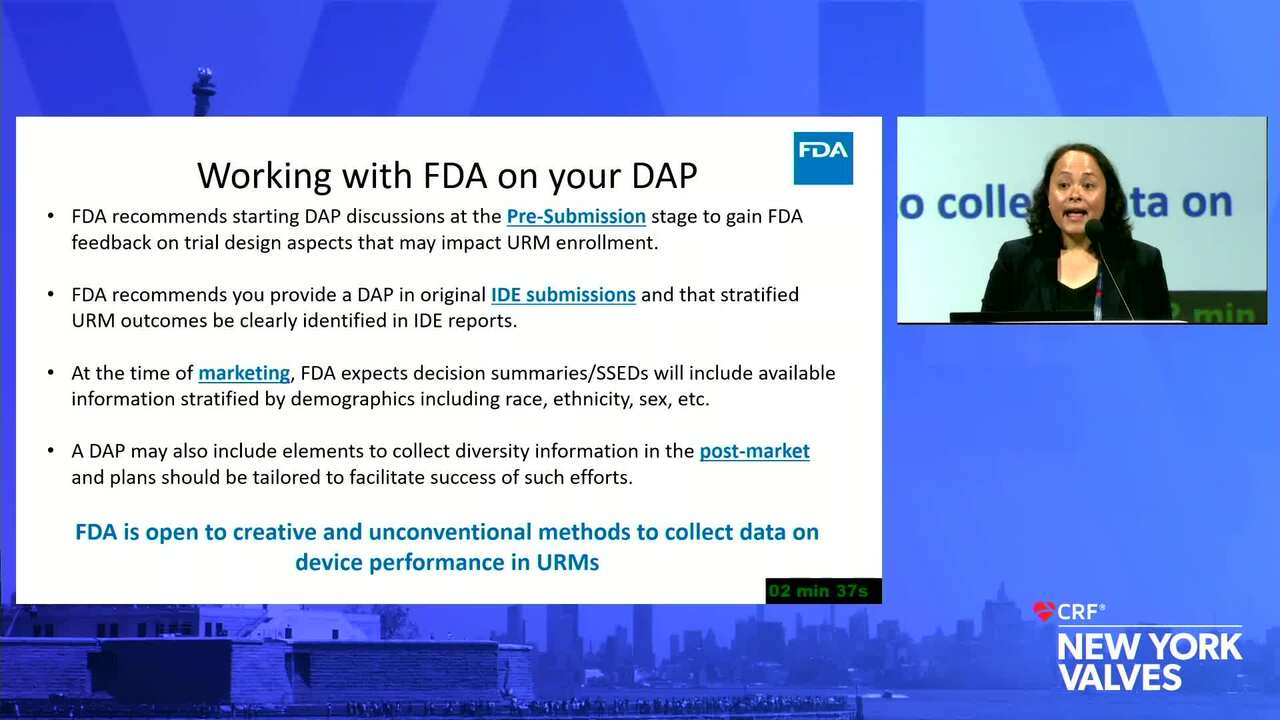
Diversity and Representation in Valvular Heart Disease: Challenges With Vulnerable, Underrepresented Populations
43 min.
Diversity and Representation in Valvular Heart Disease: Trialist Perspective
5 min.
Diversity and Representation in Valvular Heart Disease: Industry Perspective
4 min.
Diversity and Representation in Valvular Heart Disease: FDA Perspective
5 min.
Global Harmonization of Regulatory Standards
22 min.
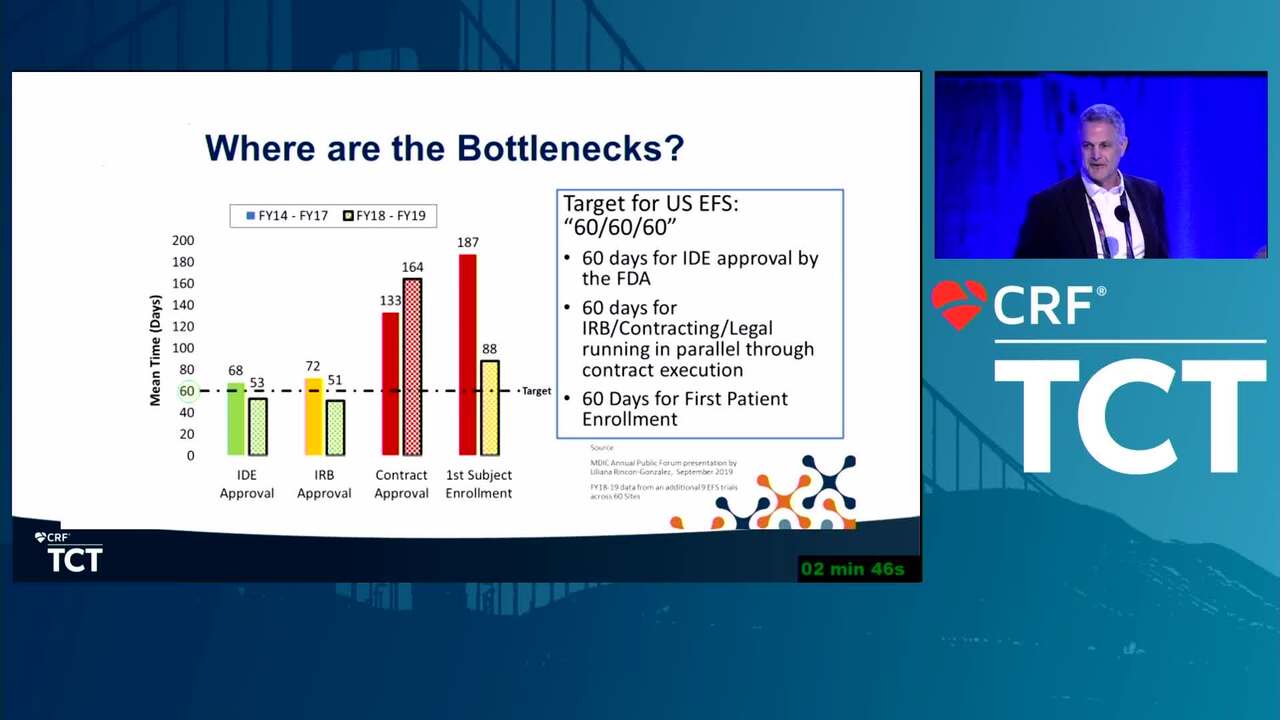
Early Feasibility Studies: How to Enable Innovation While Maintaining Safety
19 min.
Conducting Clinical Studies In and Outside the U.S.: One Journey, Two Tales
20 min.
The Lifetime Management of Aortic Stenosis: A Heart Team Perspective
8 min.
The Lifetime Management of Aortic Stenosis: The Unmet Needs
49 min.
Wearable HF Monitoring Devices: Landscape Overview, Patient Indications, and Implementation Science
25 min.
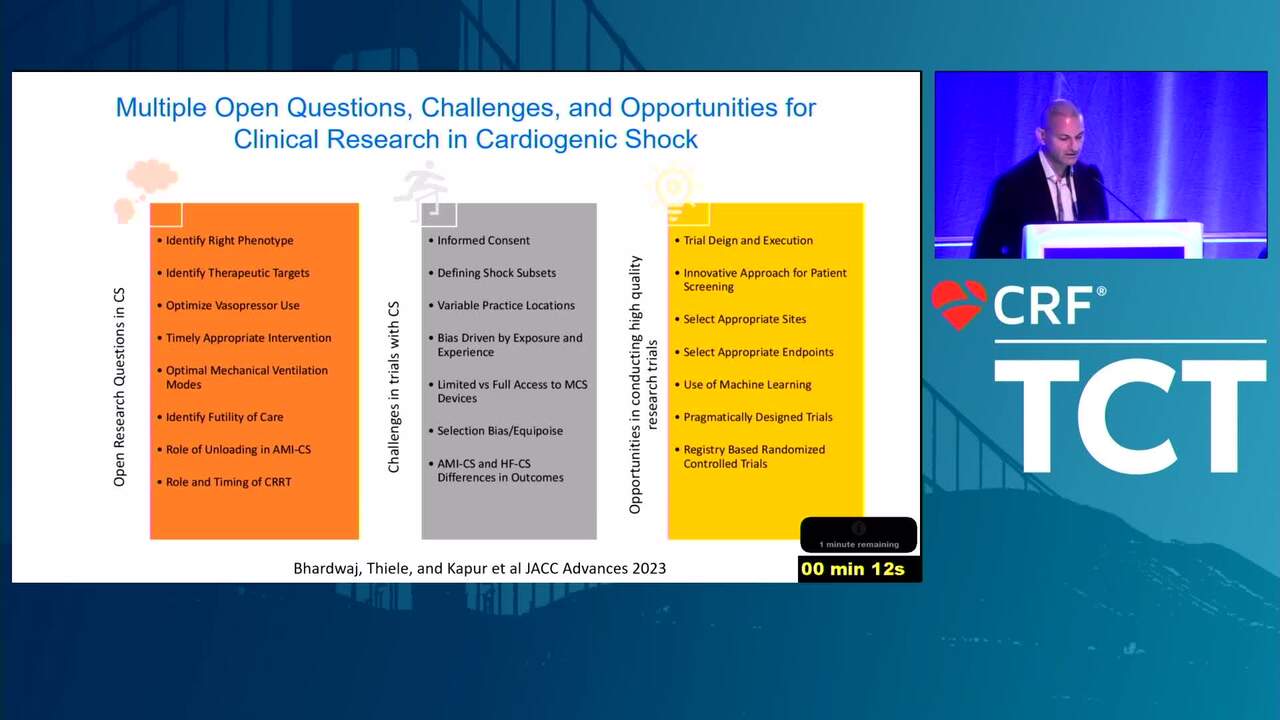
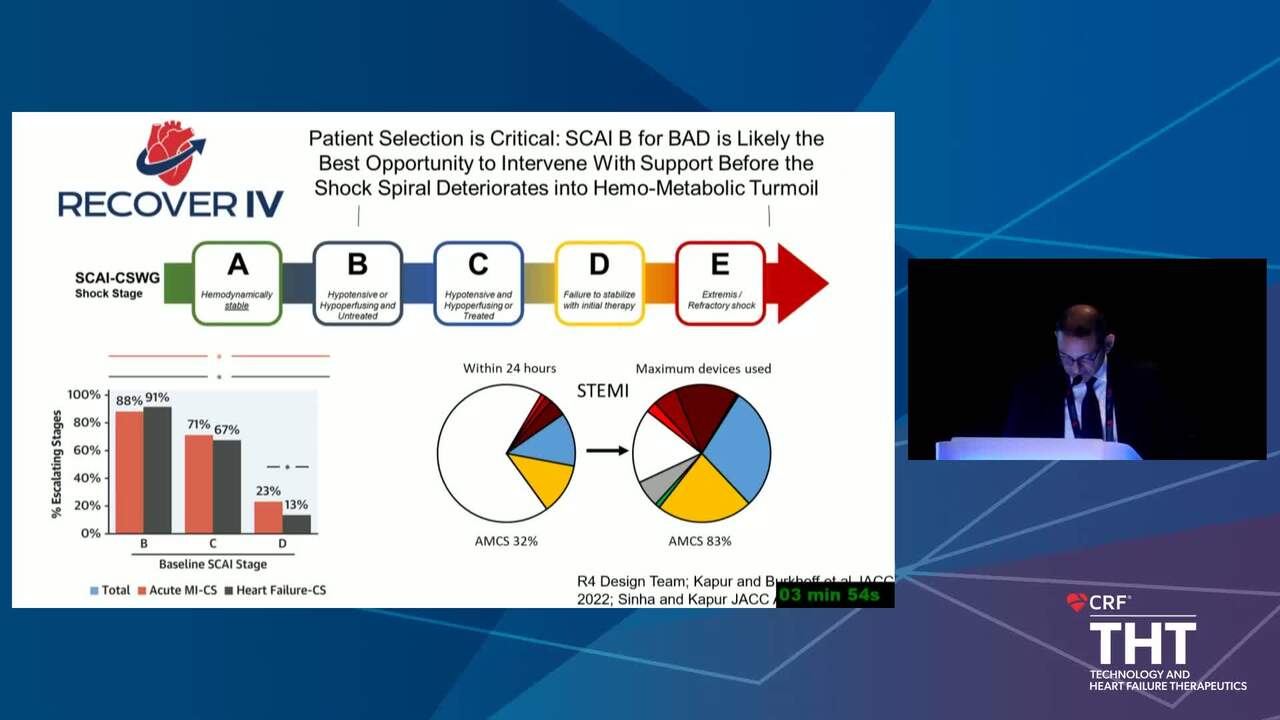
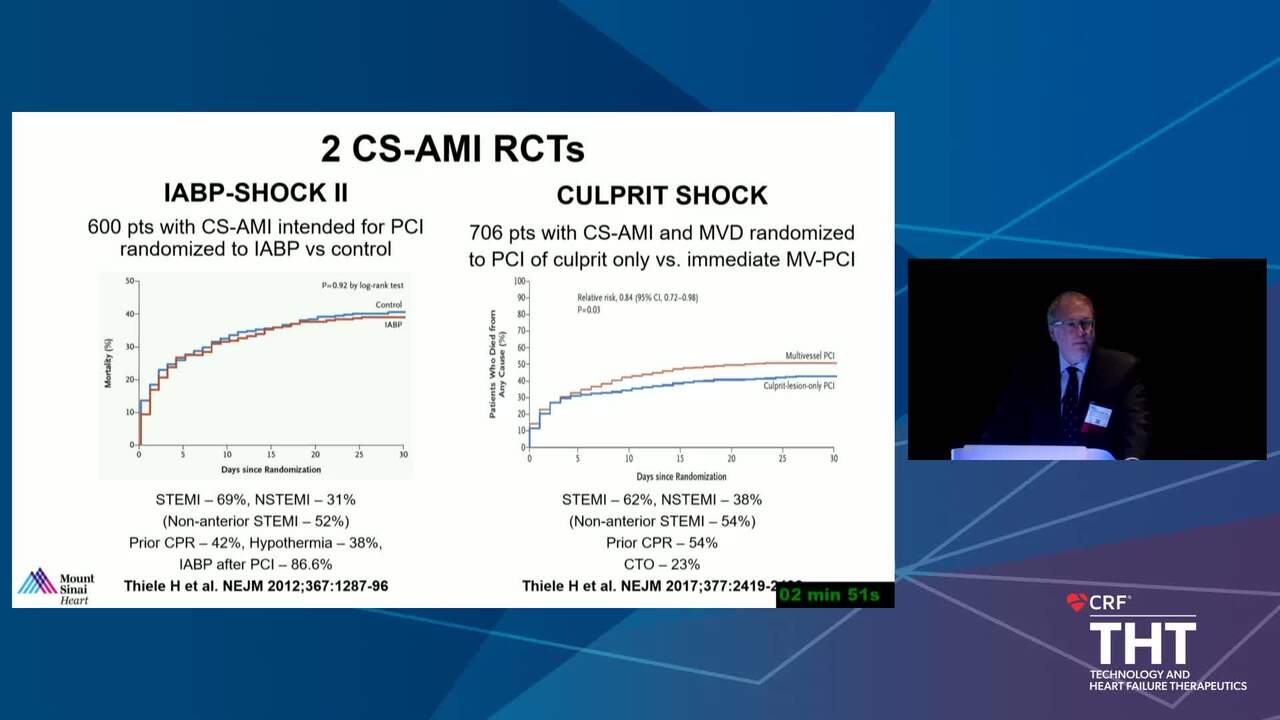
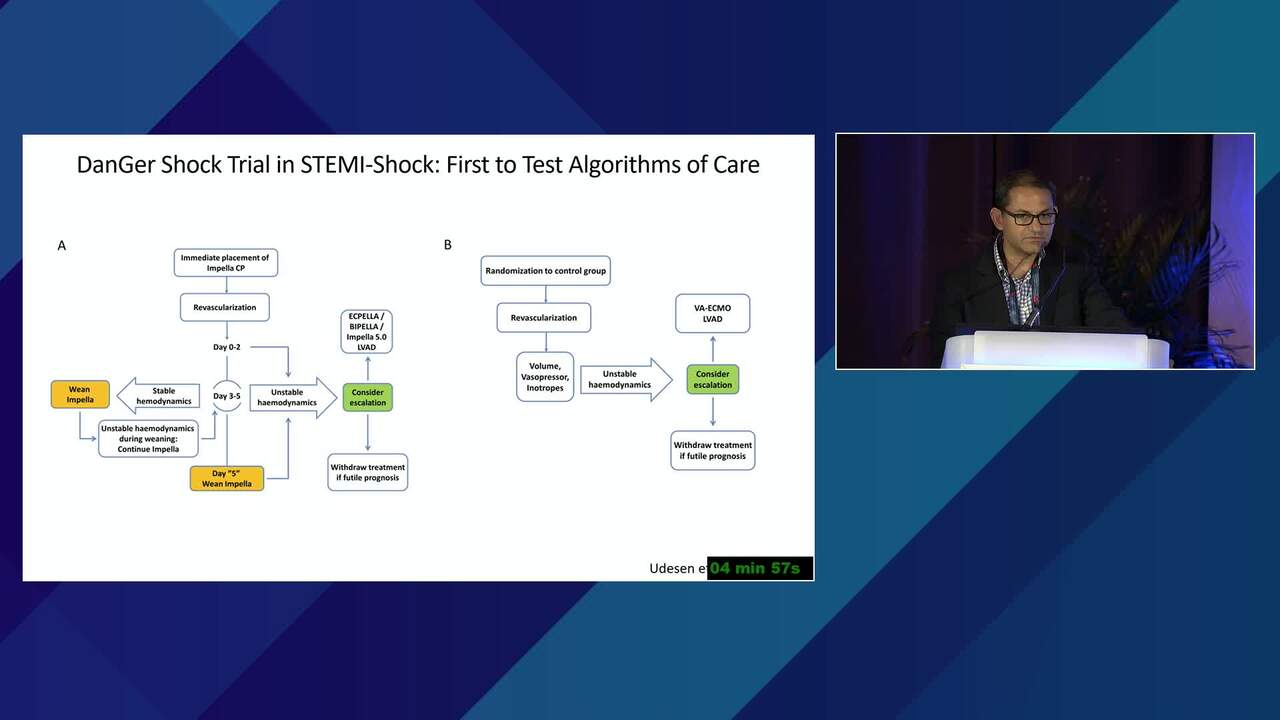
The Conundrum of Clinical Trials in Cardiogenic Shock
16 min.
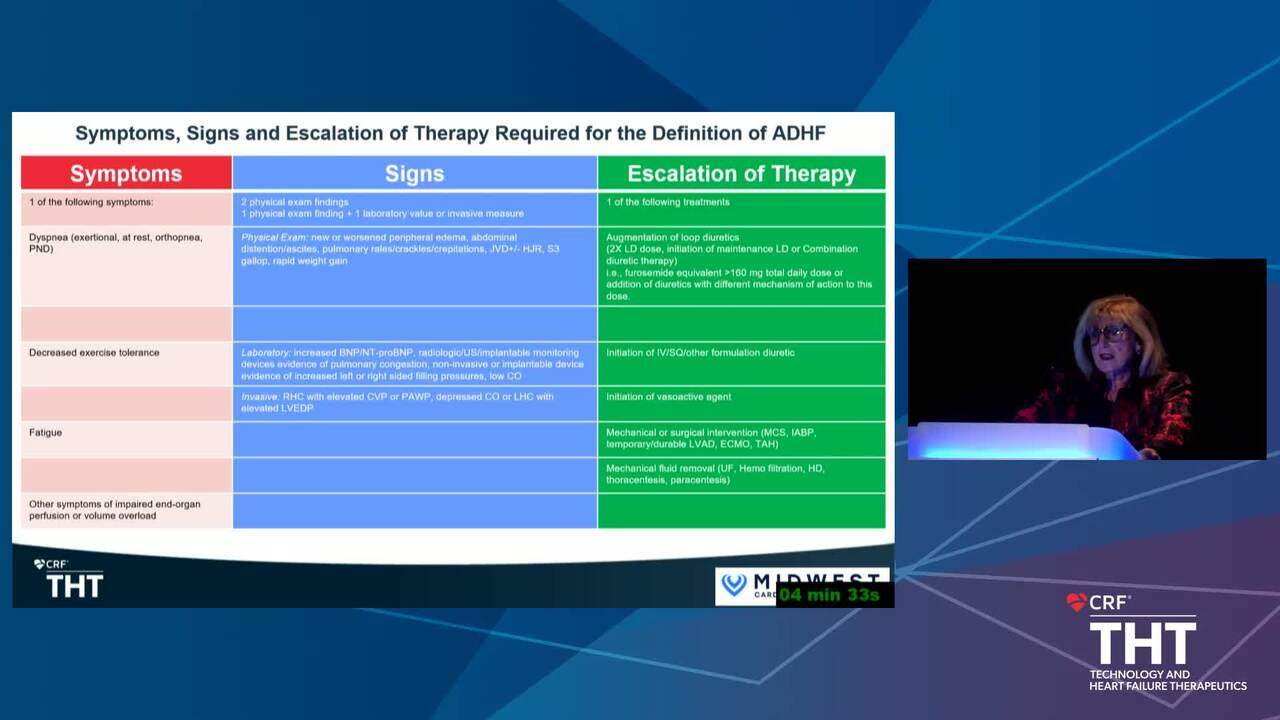
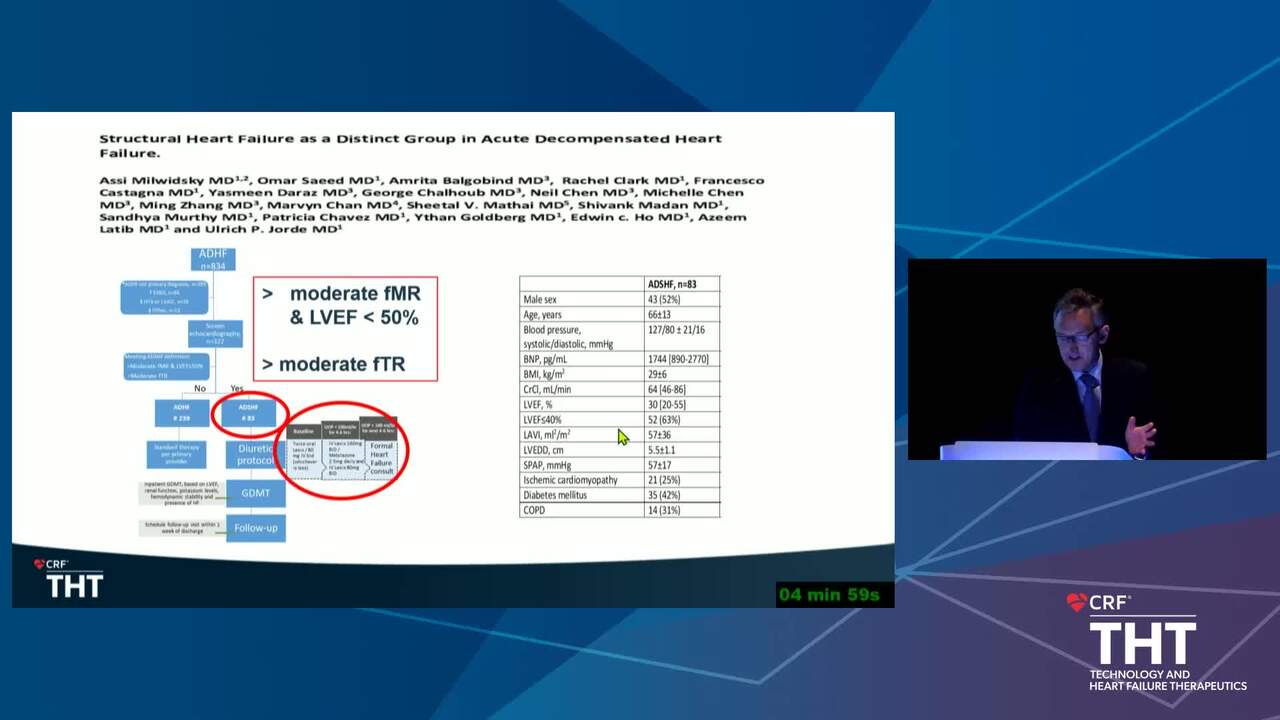
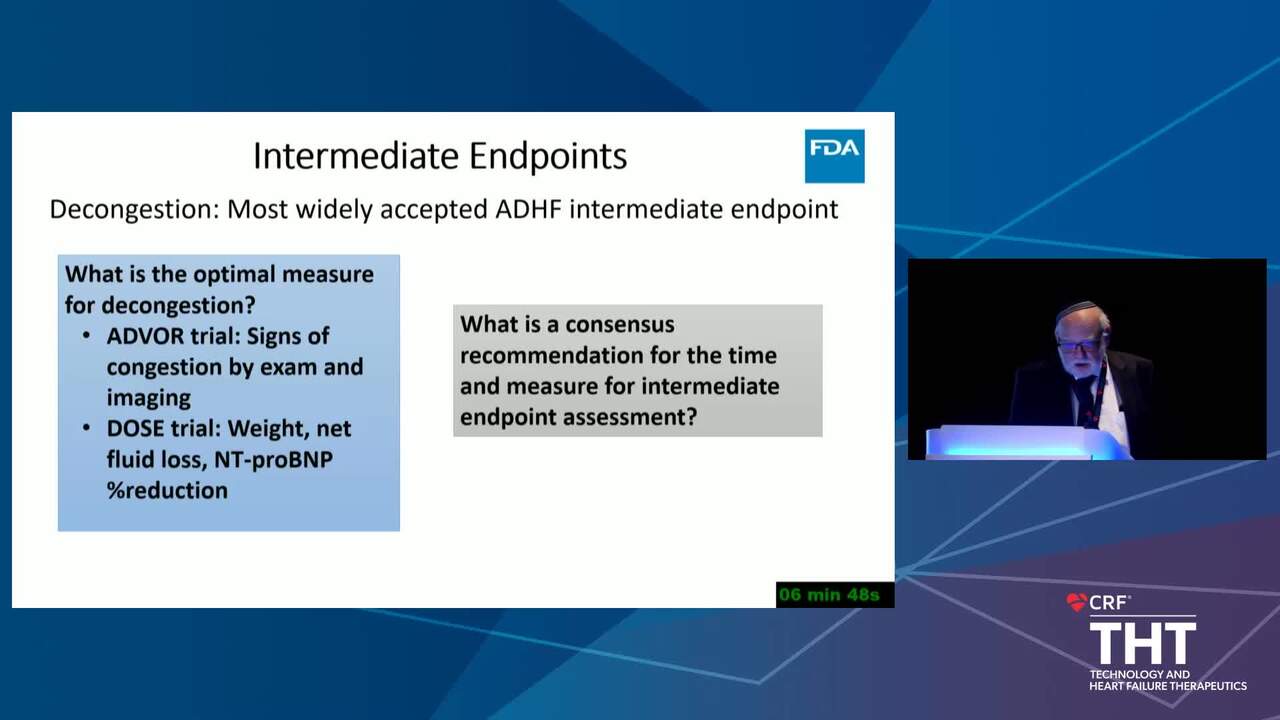
Acute Decompensated HF Trials: Who Should be Enrolled and What Should the Study Endpoints Be?
15 min.
PROs Alone in Non-Blinded Studies Shouldn’t Drive Device Approval
28 min.
PROs are a Powerful Endpoint in TR Interventional Trials
8 min.
Second Wave Technology Will Solve Most Problems – TMVR is Here to Stay in a Big Way!
16 min.
Screen Failures, Technology Woes, Lack of Clinical Evidence, and Difficult Clinical Trial Pathways – TMVR is No More than a Niche!
5 min.
What Will the Next Trial Designs Look Like for Renal Denervation?
22 min.
Renal Denervation — Clinician Perspectives
19 min.
Renal Denervation — Industry Perspectives
5 min.
Renal Denervation — Trialist Perspectives
7 min.
Clinician Perspectives
9 min.
Diversity and Inclusion: Implications for RCTs and Work Force Considerations
35 min.
FDA and Diversity Initiatives
8 min.
Industry Perspectives
9 min.
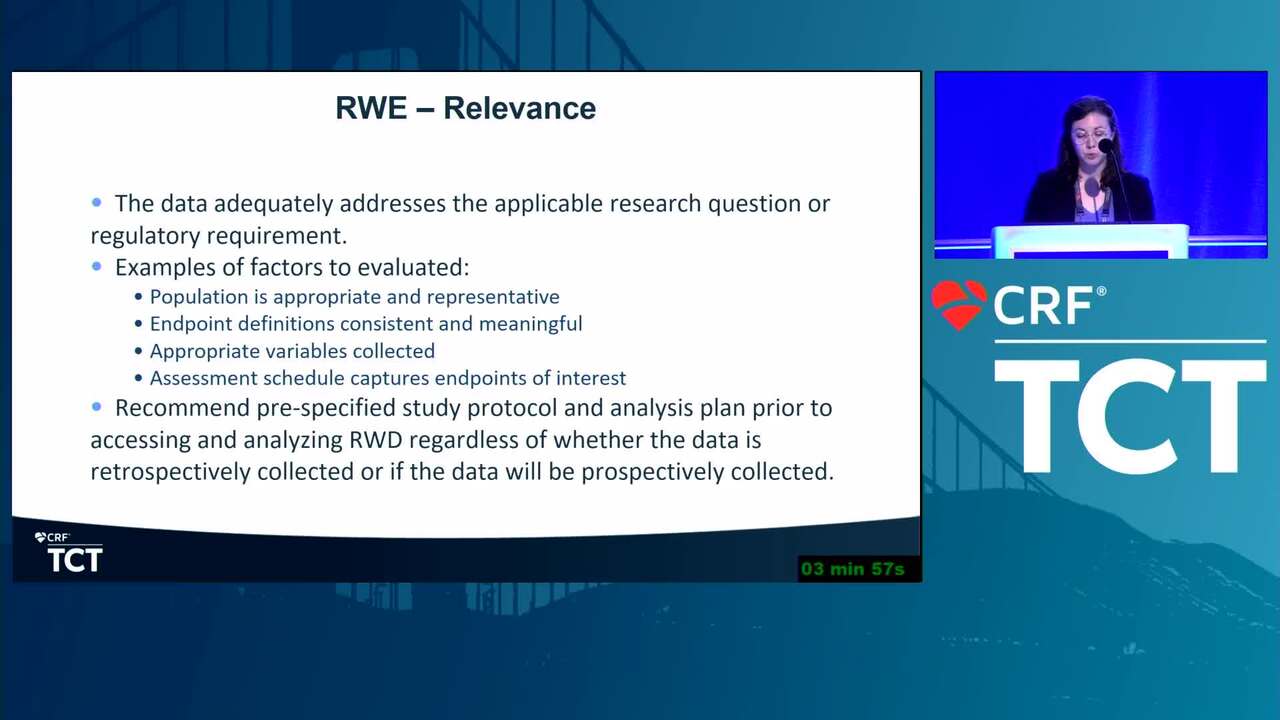
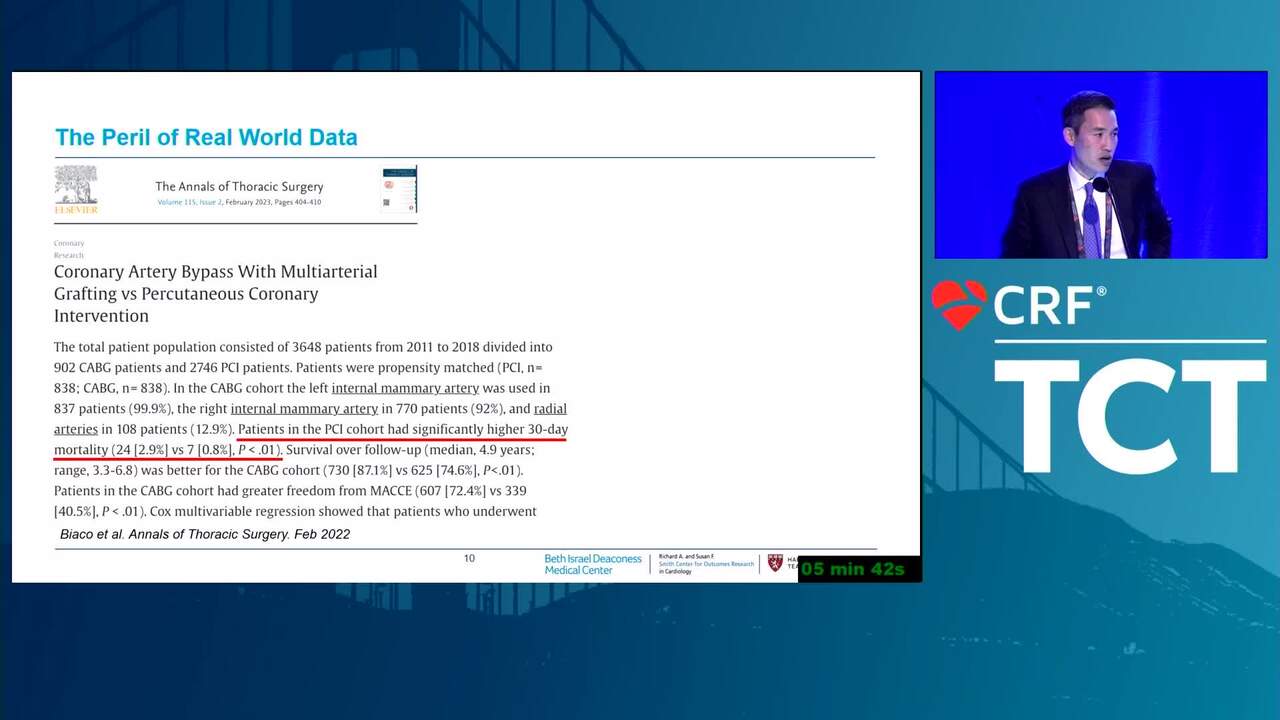
Overcoming the "Credibility Crisis" of Observational Data
13 min.
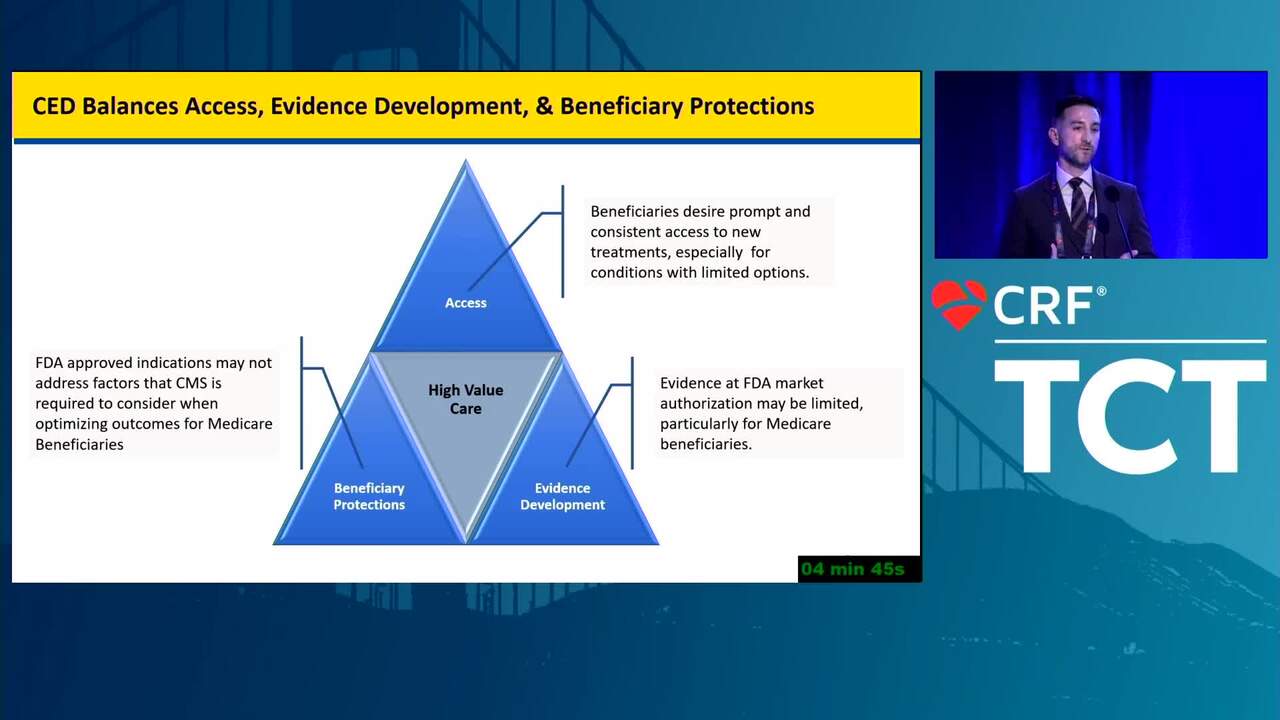
The FDA Total lifecycle Advisory Program (FDA-TAP)
36 min.
Understanding the New Technology Add-on Payments Program (NTAP)
9 min.
Status Update on the Breakthrough Device Transitional Coverage for Emerging Technologies Program (TCET)
10 min.
What Should EFS 2.0 Look Like?
42 min.
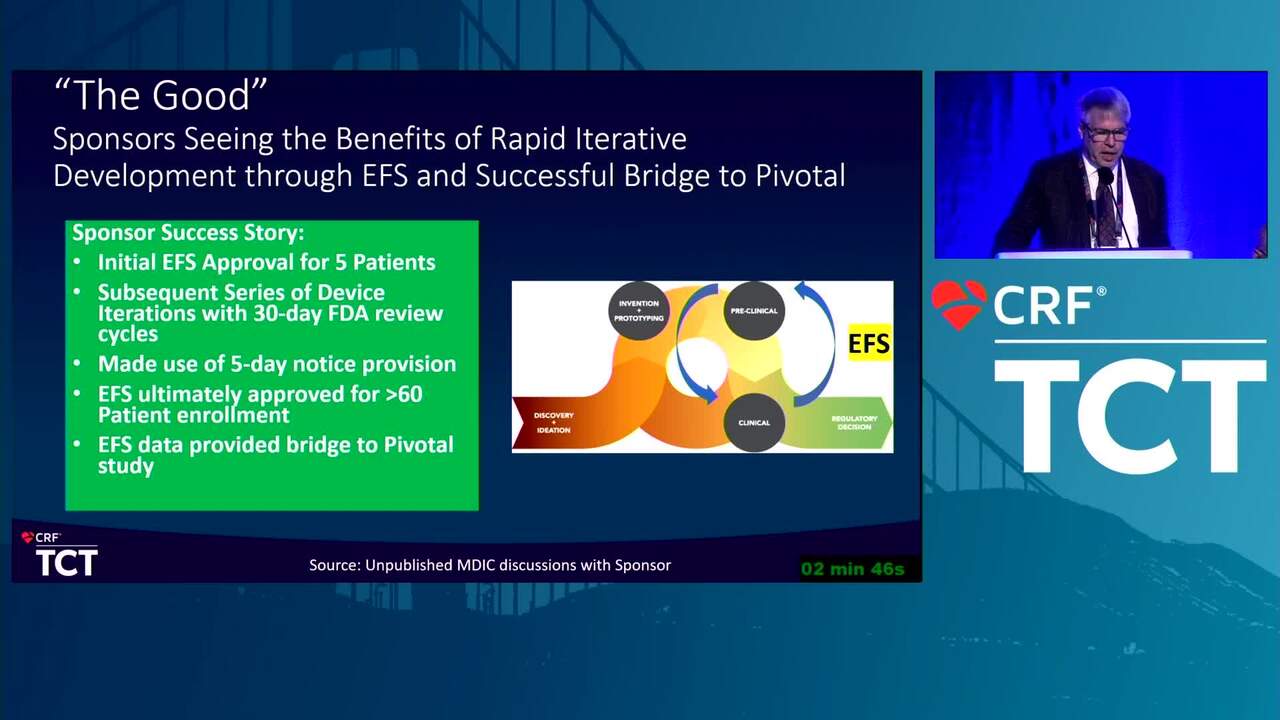
MDIC and EFS: The Good, the Bad, and the Ugly (Case Examples)
6 min.
Early Feasibility Program: Viewpoints From Europe
7 min.
EFS: Heart Valve Collaboratory
7 min.
Multiple perspectives on Early Feasibility Studies - Medical Device Innovation Consortium's Perspective
6 min.
Early Feasibility Studies (EFS) - A 10-Year Perspective - Lessons Learned
11 min.
Challenges, Opportunities, and Future Projections for Device Innovation, Approval, and Implementation - FDA Perspective
59 min.
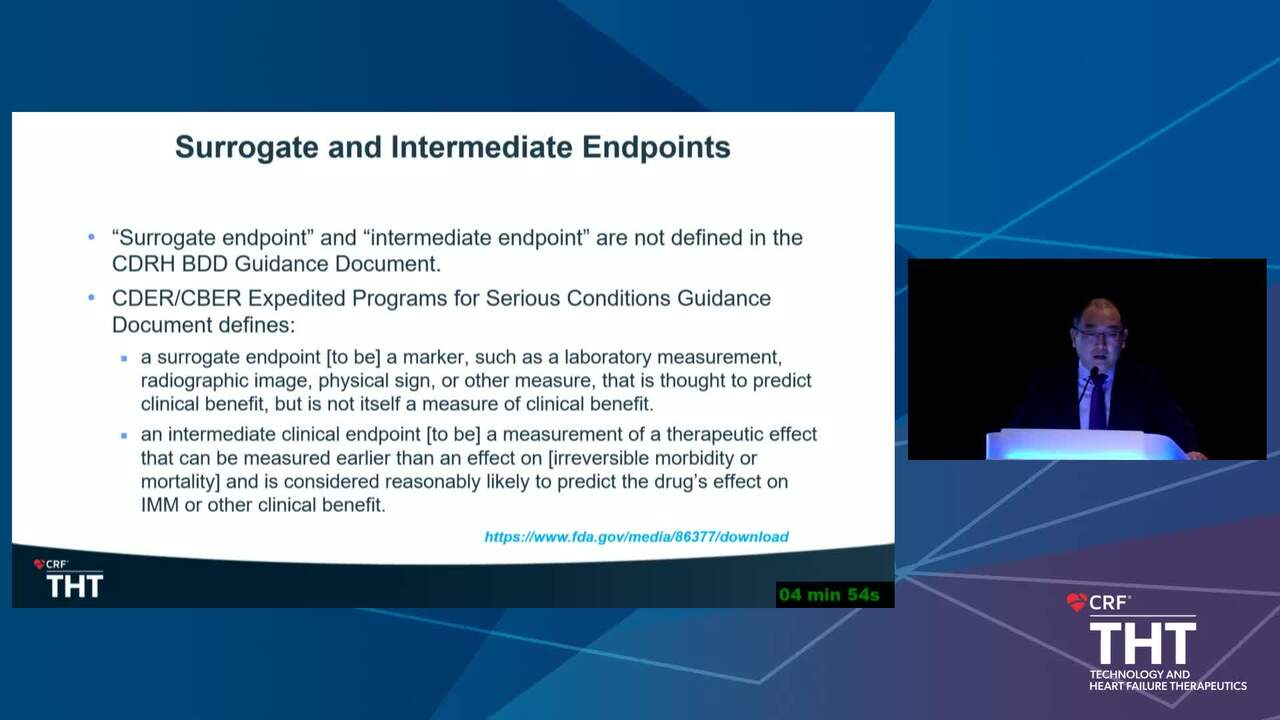
Strengths and Limitations of a Two-Phase (6-Month Intermediate Endpoints & 12-18 Month Mortality and Heart Failure Hospitalization) Design for Trials on Chronic Heart Failure Devices
10 min.
Application of Exception from Informed Consent to a Multicenter Cardiogenic Shock Trial
9 min.
Regulatory Aspects for Chronic Heart Failure Devices - FDA Perspective
10 min.
Pros and Cons of the Win Ratio as the Primary Endpoint for Pivotal Heart Failure Trial
10 min.
How Tightly Should Treatment Algorithms Be Prescribed in a Clinical Trial on Cardiogenic Shock? What Are The Key Treatment Protocol Elements?
9 min.
How Narrow or Broad Should Enrollment Criteria Be in Trials on Cardiogenic Shock to Achieve Clinically Interpretable Results?
7 min.
Clinically Meaningful and Practical Pivotal Trial Endpoints for Devices in Acute Decompensated Heart Failure
9 min.
Invasive Investigational Device IDE Studies for Acute Decompensated Heart Failure: For Diuretic-Resistant Patients Only?
9 min.
Regulatory Questions on Device Trials for Acute Decompensated Heart Failure - FDA Perspective
6 min.
Early Feasibility Studies (EFS) “2.0”: The Next Decade
26 min.
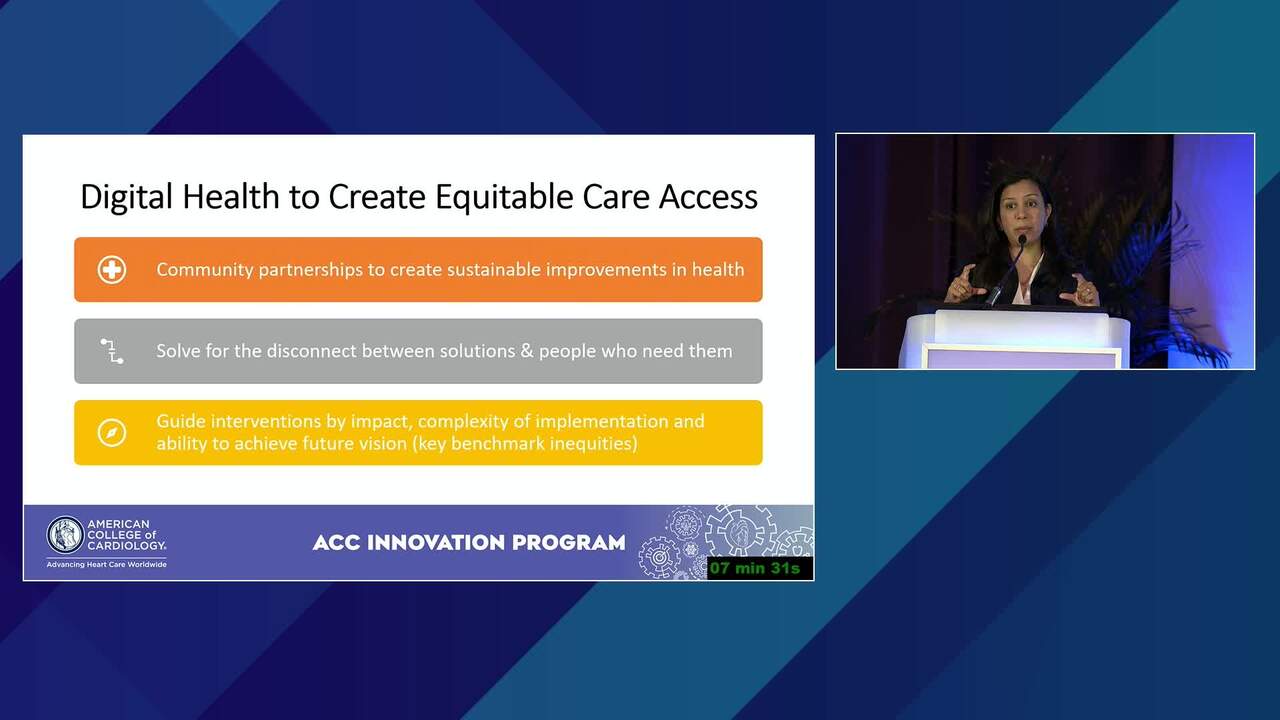
The Transformative Potential of Digital Health Strategies to Impact Clinical Research and Patient Outcomes
15 min.
The Importance of Pre-emptive TAVR in the Future Management of Aortic Stenosis
13 min.
Strengths and Limitations of Patient Reported Outcomes as Major Endpoints in "Modern Era" CV Trials
12 min.
Cardiogenic Shock Trials: Defining the Clinical Need and Framing the Issues
10 min.
Transformative Changes in Clinical Research: A Futurist’s Viewpoint
16 min.
Health Equity Issues in Clinical Trials: Can We Accelerate Progress?
15 min.
FDA Recovering From a Pandemic: Lessons Learned and Future Adjustments
10 min.
FDA Update on EFS Requirements for TTVR Technologies
9 min.